

Footnotes
cUTI: complicatedurinary tract infection; NTM-PD: non-tuberculous mycobacterial pulmonary disease;

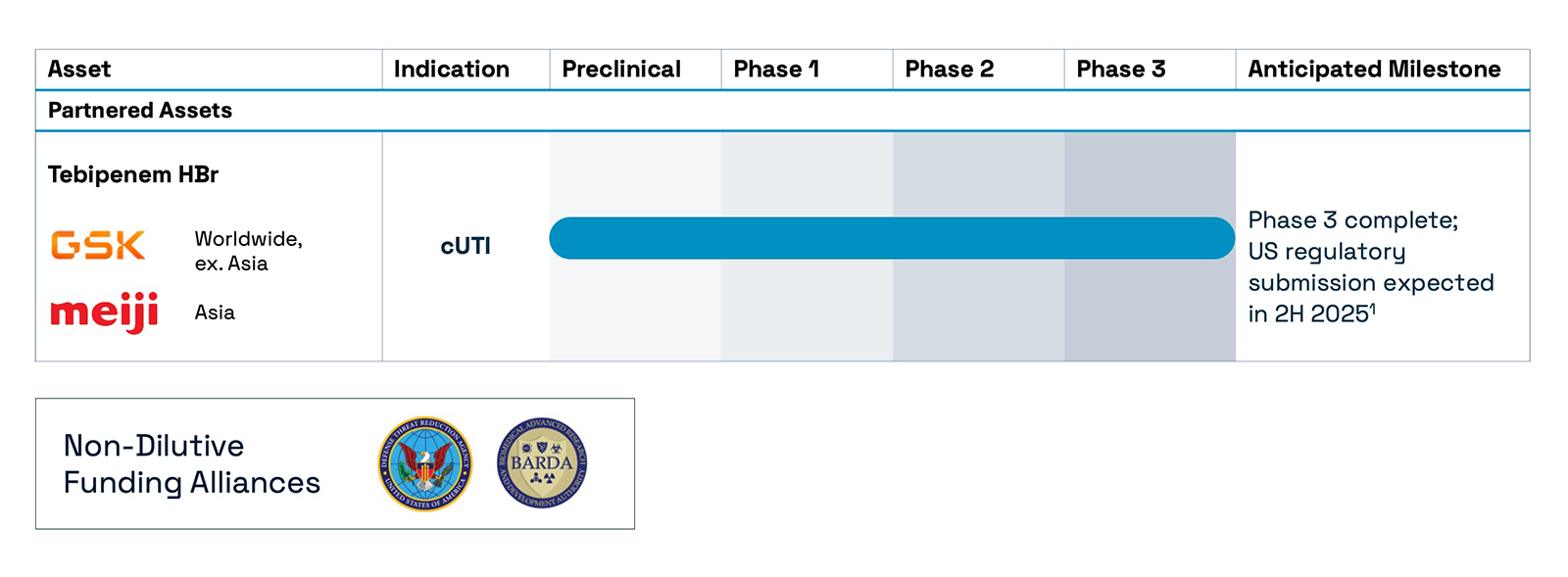
An investigational oral carbapenem being developed for the treatment of complicated urinary tract infection (cUTI) including pyelonephritis.
Tebipenem HBr is an investigational, novel, oral antibiotic designed to tackle cUTIs including pyelonephritis. Tebipenem HBr belongs to the carbapenem class, which inhibits bacterial cell wall synthesis. If approved by the FDA, it could be the first oral carbapenem available in the U.S. Spero granted GSK an exclusive license to commercialize tebipenem HBr in all territories, except certain Asian territories.
The pivotal PIVOT-PO Phase 3 clinical trial, which began enrollment in January 2024, was designed to evaluate tebipenem HBr in patients with cUTI, including pyelonephritis. Following a pre-specified interim analysis, an Independent Data Monitoring Committee (IDMC) recommended stopping the trial early for efficacy. The analysis confirmed that tebipenem HBr met its primary endpoint, demonstrating non-inferiority to intravenous (IV) imipenem-cilastatin in hospitalized adult patients with cUTI, including pyelonephritis. No new safety concerns were identified beyond previously reported adverse events.
GSK plans to submit the trial data as part of a planned US Food and Drug Administration (FDA) filing in 2H 2025. If approved, tebipenem HBr could change the treatment landscape for cUTI patients, offering a much-needed oral alternative to IV carbapenems.
For more information on the trial, see ClinicalTrials.gov identifier NCT06059846.
Spero Therapeutics believes that forming collaborations with external partners is essential to maximizing the reach and impact of our innovative therapies for patients.
Stock information, SEC filings, corporate governance, IR resources and more.
Join our life-changing mission to deliver differentiated treatments to patients suffering with rare diseases and MDR bacterial infections. We seek individuals with unique talents who share our passion.